學會會訊 2014 - 02
第六屆華夏論壇於香港沙田圓滿成功!
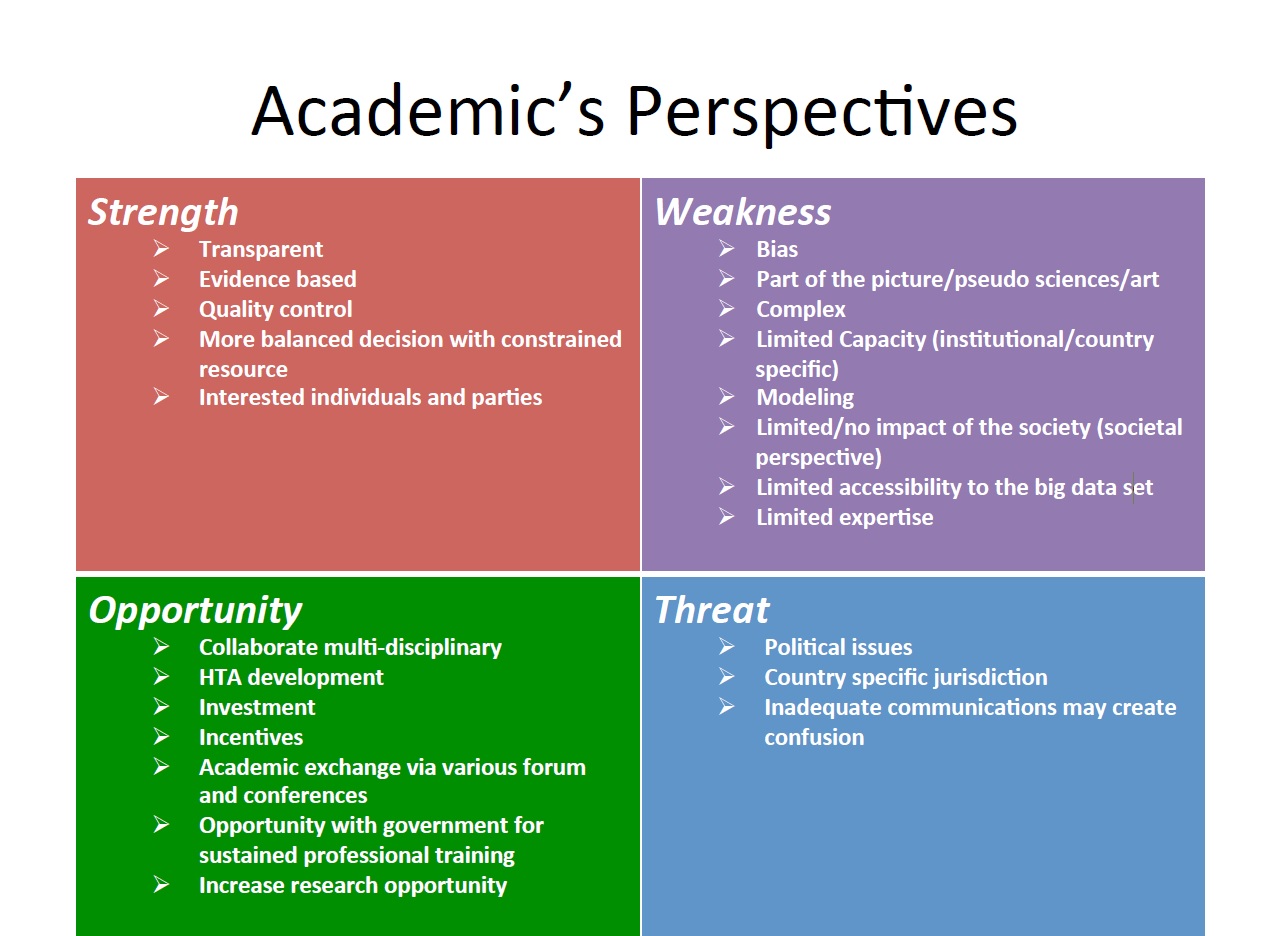
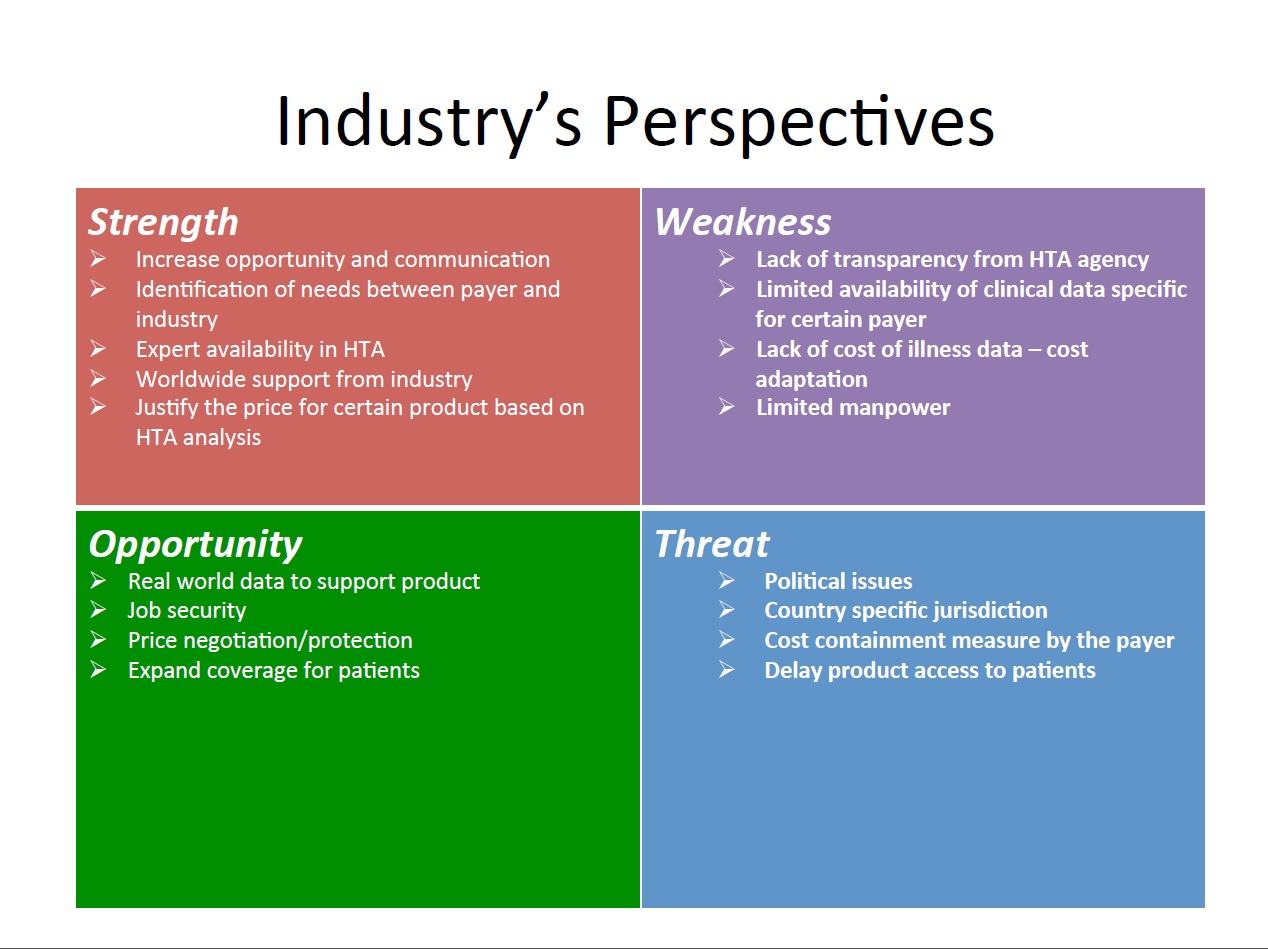
2014年第六屆華夏衛生科技評估論壇於香港沙田圓滿落幕,本期會刊我們邀請了三位與會會員與大家分享心得與所見所聞。並以三張SWOT分析表摘錄兩天會議的重點精華,進行新知分享與交流。


20104年華夏論壇與會心得分享
譚家惠 博士 / 台灣大學健康政策與管理所 博士後研究員
其中,李樹泉教授針對「What is an appropriate threshold for Asia」提到各國對於ICER threshold quoted,例如美國以US$50,000/QALY、加拿大以CAN$20,000-100,000/QALY、澳大利亞的PBAC以A$36,000-50,000/QALY、英國NICE以₤20,000-30,000/QALY作為threshold,而WHO對於發展中國家,則建議以該國1 to 3 per capita GDP/DALY做為threshold。然而,影響ICER threshold的因素眾多,包括外在環境的政治、科技、環境因素,以及病人本身的疾病,與藥物本身的因素共同影響;其次,對於新的醫療技術、新藥本身HTA決策,對於健康保險給付的影響,已在過去有諸多研究探討,民眾本身的願付價格(Willingness to pay, WTP)受到其年齡、性別、社經地位、健康狀態等因素的影響,因此要定義出健康保險給付決策的閥值,是有其困難度;因此,threshold應在不同給付者(payer)、不同族群(population),甚至不同處置(procedure)間具有差異。
因此,缺乏單一及被普遍接受的健康收益閥值(health gain threshold)是當前評估健康介入以及衛生資源分配的一大障礙,雖然以per QALY gain等相同尺度為比較基礎的閥值(threshold),則可協助衛生資源分配決策,閥值不過是衛生決策的參考因素之一,且在具有不同負擔的疾病間,需要不同的閥值。然而,無論從技術或理論層面上來看,目前沒有充分的數據可以提供單一通用於不同疾病間與不同處置間的單一ICER threshold,以實證資料來看,若以民眾端或病人端訂定處置或介入的成本效益閥值,其可能遠低於目前健康保險給付的價值,且若選擇單一閥值,則可能導致潛在的預算控制與資源分配不合理的問題。
本次大會主題在於交流亞洲地區的HTA資訊,以各國的HTA政策對於產業的影響,以韓國為例,整體HTA制度的主要目的乃提升資源分配效率,目前韓國有逐步減少審查時間,關於是否收載的Health Insurance Review and Assessment Service時間是120天內,而NHI服務的議價則是約60天,取得衛生福利部核可是30天,並依據疾病有訂有彈性的ICER閥值。
台灣的HTA自2007年成立,並於2008年開始協助健保署執行新藥收載與新醫療科技給付的評估工作,於2013年成立國家醫療科技評估中心(NIHTA),目前執行方式為NIHTA在42天內以正式公函將評估報告(包括臨床療效評估與經濟評估)寄送給健保署,健保署接到評估報告後,將申請案件與連同評估報告提至全民健康保險藥物給付項目及支付標準共同擬定會議上討論,若提出之本土經濟評估報告品質良好,則最高可額外10%加成藥價。
泰國自2007年起Nation List of Essential Medicine(NLEM)針對國家重要藥品採行HTA方式,特別就效用、安全、成本效益和預算衝擊進行分析,由NLEM的健康經濟工作群支援,大部分評估係由Health Interventions and Technology Assessment Programme (HITAP)進行,少部分由學界及藥界企業進行;雖然每年投入一百萬美金,但仍缺乏本土性數據。
日本HTA系統於1961年成立,新科技、藥品及設施的同意、給付及訂價是由Ministry of Health, Labor and Welfare(MHLW)決定,且採Quasi-VBP in Japan定價方式,係以兩年內依實際經驗之臨床效益,並考量價量之預算衝擊,對控制價格增加獎勵金方式,每兩年修訂一次價格;1992年起,新藥申請建議可附上經濟評估報告,但非強制性,目前日本核價過程乃評估其品質、安全性及效用(efficacy),成本效益分析雖然被建議納入考量,但尚在討論當中。日本HTA當前問題在於沒有足夠的流行病學或健康照護成本資料可進行分析。
最後,實施HTA可能帶來的潛在缺點,包括:
1. 延後病人藥品的可近性:準備HTA報告、審核以及評估的時間,約40到210天。
2. 增加決策的成本與複雜性:HTA所需的評估技術、評價,以及能力建構,以及資料來源所需的資料庫與人力資源等,都會增加HTA執行的潛在成本。
這些成本與耗用的時間的價值,是否有反映在核定的價格,以及未來運用於健康照護體系之內,乃值得深入探討。
第六屆華夏論壇心得分享
歐凰姿 博士 / 成功大學 成大臨床藥學與藥物科技研究所 助理教授
The 6th Huaxia Health Technology Forum was held from April 26-27 2014 in Hong Kong and co-organized by International Society for Pharmacoeconomics and Outcomes Research –Hong Kong (ISPOR-HK), and School of Pharmacy, Faculty of Medicine, the Chinese University of Hong Kong. The attendees from the government agencies, academics and industries have all come to be part of the Huaxia Forum from major countries in this region including Taiwan, China, Hong Kong, Macau, Malaysia, and Australia. Hereby, remarkable contributions from all the participants led to the substantial success of this Forum.
The Huaxia Forum this year raises a theme of “Health Technology Assessment (HTA)– Impact on Health Care and Assessment of the Values of Medicines.” The Forum was a unique venue for learning and exposing to the most current issues related to the adaptability of HTA in Asia, and for recognizing potential challenges in health and medicine sector, strategies and actions in the implementation of HTA in Asia.The Forum also served a platform where pharmacists, pharmaceutical scientists, decision-makers, manufacturers and educators in Asia can meet, learn, share and exchange views.
The first day of the Forum was opened by Professor Sophia Chan (JP, Under Secretary for Food and Health Government of the Hong Kong Special Administrative Region). She gave an excellent keynote on healthcare reforms in HK and provided one example of recent initiatives – the Health Protection Scheme (HPS)”. The forum also went through one critical issue -“What is an Appropriate Incremental Cost-Effectiveness Ratio (ICER) Threshold for Asia?”, presented by Professor Shu-Chuen Li (Chair Professor and Head, Discipline of Pharmacy & Experimental Pharmacology, School of Biomedical Sciences, University of Newcastle). The presentation gave an overview of different ICER thresholds being used across Asian countries and discussed the various issues related to the formulation of ICER threshold. The empirical evidence demonstrated that the ICER threshold used in Asia varied by countries and diseases, and were much lower than that recommended by the World Health Organization (two to three times Gross Domestic Product per capita). Although the use of explicit threshold value has the advantage of transparency and facilitates resource allocation, critical questions still remain in Asia-“is one standard value that can be used globally or within one country ?” and ”if so, how the standard should be established?”
An interesting discussion - “What is the Impact of HTA on healthcare” was brought by several speakers from pharmaceutical industry and academics. From the discussion, we recognized that today countries around the world are striving to achieve universal health coverage. This means that all people are able to access and use the health services they need without suffering financial hardship. A major challenge is to ensure equity, quality of care and efficiency. In pursuit of achieving such coverage and ensuring provision of affordable services and best use of resources, one of the tools countries is using HTA.Although HTA has been used as a tool in evaluating new technologies for a few decades, it is still under active development to meet the new challenges brought by the advent in research, the invent of new modalities, the unmet expectations of stakeholders and the efficiency of health care systems. HTA is a process which may involves many dimensions and with different perspectives for different stakeholders. The applicability of the outcome of this process may vary with time, systems and models. It only contributes part of the information required in the whole basket of factors which have to be considered in the decision making process.
The role of patient in HTA was focused on the second day of the Forum. The topics of“Why Should Patients Be Involved in Health Technology Assessment to Assess the Value of Medicine?” and “How does Patient See the “Value of Medicine?” advocated for patient involvement to defining and providing patient evidence for decision-making. It is important to recognize that decisions which affect people live take into account the needs of those that will be affected. Therefore, patient and public input can, and should help determine whether treatments, including new treatments, are available. Patient-based evidence is one of three pillars of evidence in HTA, but it is conceptually and methodologically less well developed internationally. Patients need to be involved in shaping evidence. The unique contribution of the evidence from patients answers questions of appropriateness, effectiveness, acceptability and relevance from patients’ perspective and reflects the reality and complexity of healthcare.
Dr. Tony Yen-Huei (executive director, Center for Pharmaceutical Care Development, Taiwan Pharmacist Association) also shared Taiwan’s experience on the second day. His topic of “What is the Value of Medicine in Taiwan?” stand from a public health service provider’s perspective such as Taiwan’s National Health Insurance Administration (NHIA) to appreciate the applicability and efficiency of HTA in Asia.
Several interesting talks from individual countries were presented as empirical evidence of assessing the Value of Medicines in Asia, including “Assessing Outcomes via Disease Registry – a 6 year Cardiology Experience in Malaysia,” “The Ups and Downs to Assess the Value of Medicines in Mainland,” and an overview of the challenges of vaccine HTA in Asia.
The journey to dig into the wealth of HTA is like an exploration trip. It sounds exciting and full of unknown challenges. HTA is an essential factor to be considered as the organization is under public scrutiny. The adaptability of HTA to the pace of advancement in health technology and to the clinical development is like a difficult situation for which there is no easy or possible solution. Do we need to conduct HTA on the same topic regularly to take into account the applicability of the last HTA outcome to the constant changing environment? Is evaluation of last HTA after certain period of time necessary and cost-effective? Experiences in HTA application from overseas’ authorities are invaluable to a public service provider like Taiwan’s National Health Insurance Administration.
Finally, I am very thankful for this opportunity and look forward to attending the Huaxia Forum in the near future. Hope that the participation in the Forum will impact on my scholarly career and pharmacy communities in Taiwan.

第六屆華夏論壇紀實
李振宇 博士 / 長庚大學臨床資訊與醫學統計研究中心 博士後研究員
今年(2014)季春在香港沙田所舉行的第六屆華夏論壇已圓滿結束。在這兩天一夜的行程當中,除了體驗到這人文薈萃、經濟繁榮的美食天堂都市之外,再來就是參與聆聽亞洲華人地區對於衛生科技評估(Health Technology Assessment)領域的努力與成果。期間有來自中國、香港、澳門、新加坡、馬來西亞與台灣等官方、學界與業界的專家學者分享當地實施HTA的經驗。受限於地區的環境因素,各地施行HTA所遇到的困難或瓶頸,在會場都可以討論並尋求解決之道,大致上都得到相對應的回應。
HTA在台灣地區由於譚教授與各方先進學者帶領下已見成效且頗具規模。然而台灣地狹人稠的地理情況,對於傳染病的流行仍需戒慎恐懼。施打疫苗是一種很有效去預防感染傳染病流行的方式,但評估施打疫苗後的效果一直都是研究人員想要詮釋的議題。施打疫苗者除了可以讓自身得到某時段內的保護力,降低傳染病感染的風險,也可以間接減少周遭未施打疫苗者受感染的機率。會中講者吳博士藉由與張教授所發展的動態傳染病模型可以適時地反應出傳染病感染與免疫效果的情形。另外,由於流行性病毒會持續改變型態且疫苗的成本仍高,在政府財務預算有限下,如何正確地分配施打疫苗者優先順序,這也是一件克不容緩的議題。
最後,謝謝大會提供精彩的演講、豐富的餐點與舒適的環境,謝謝。


秘書處公告
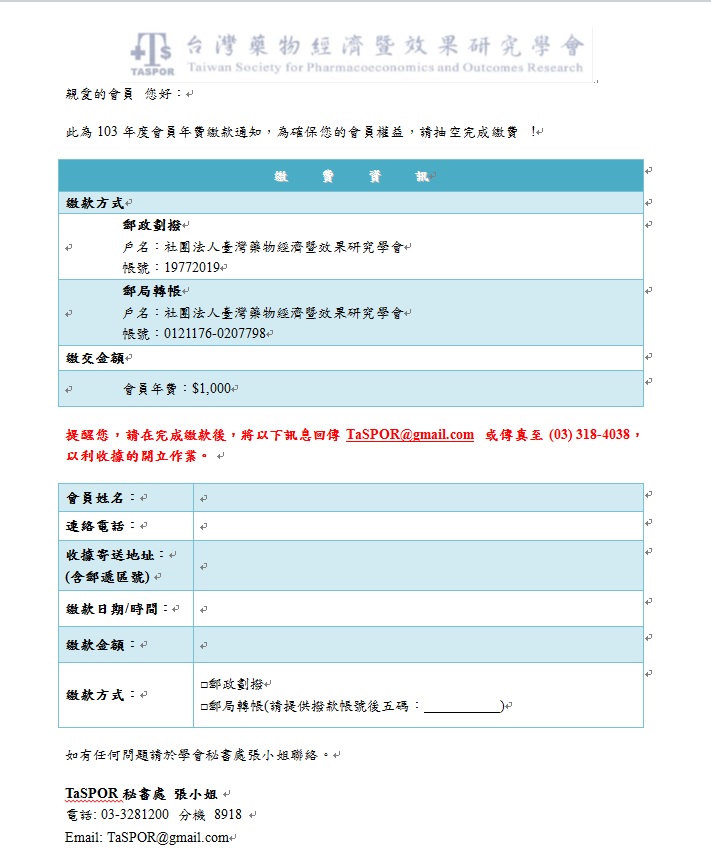
1. 103年度會費已開始繳納,請尚未繳納之會員至郵局轉帳或劃播,謝謝 。
2. 課程訊息
[ Outcome Research using Big Data Workshop 即將在6月23日假台大醫院國際會議中心203室舉行 ]
本會會員免費,報名位置 : http://www.taspor.org.tw/course_02.php?id=18
[生物相似性藥物之探討 Biosimilars or Biobetters 即將在6月28日假台北國際會議中心3F南軒舉行]
時間:103年6月28日(六) 上午 8:30~12:00
地點:台北國際會議中心3F 南軒
聯絡人: 中華醫學會 余小姐,02-28757358。
聯絡人: 中華醫學會 余小姐,02-28757358。
3. 歡迎新會員入會
| 會員編號 | 姓名 |
| 1395 | 蘇健智 |